Life today is electronic; connected; made possible by devices which bring information at our fingertips (or rather mobile screens) when and where we want them. For all the power and promise of these miraculous- and now ubiquitous devices, their effectiveness is stymied by the most basic (and oldest) of problems.
Energy!
Modern devices have to maintain their lilliputian size to please the modern consumer and for practicality of mobility, but this comes at the price of smaller and lower capacity battery. Lithium polymer batteries are the latest in battery tech, but they also collide against their limitations when faced with the onslaught of the power hungry and demanding nature of today's consumers. These batteries have high energy densities, capable of providing energy slowly, for hours at end, but they also take a long time to charge and have less power. Capacitors are small devices which can hold electrical charge. They charge up quickly and deliver that in a single powerful burst at a precise moment. Camera flash is the easiest example which comes to mind.
Batteries have a positively charged anode, electrically linked to a negatively charged cathode via a conducting solution called an electrolyte. Positively charged ions move from anode to cathode, generating a current to power our devices, while recharging moves these ions back to anode. An ideal battery would be something which would combine the rapid charging and high power output of a capacitor with the energy density of a battery.
A research group in MIT may have answered our digital prayers, they managed to upgrade the cathode to a whole new level using carbon nanotubes- single molecule thick tubes of carbon- coated with a chemical which gave them a negative or a positive charge. The researchers dipped a base substrate material alternately into a positive and negatively charged carbon nanotube solutions. The oppositely charged layers allowed the nanotubes to successfully self-assemble into monolayers rather then getting clumped together (link to article).
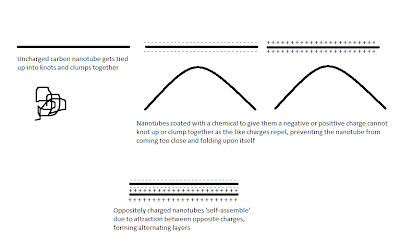 This ensured that the nano-scale porosity of the carbon nanotubes was maintained, making a large number of oxygen atoms accessible to incoming cations. Along with the very high conductivity of carbon nanotubes, the cathode could accept a large number of cations and move them very quickly, generating a large amount of power and retaining the energy capacity of a battery. The newly fangled contraption gives energy output 5 times that of a capacitor at a power delivery rate 10 times that of a lithium ion battery.
This ensured that the nano-scale porosity of the carbon nanotubes was maintained, making a large number of oxygen atoms accessible to incoming cations. Along with the very high conductivity of carbon nanotubes, the cathode could accept a large number of cations and move them very quickly, generating a large amount of power and retaining the energy capacity of a battery. The newly fangled contraption gives energy output 5 times that of a capacitor at a power delivery rate 10 times that of a lithium ion battery.Several problems remain though, for rapid commercialisation. For example: The researchers created the electrode manually, with a 3 micrometer thick electrode consuming 8000 minutes to make. Scaling up of the technology in a rapid yet cost-effective manner is necessary and is underway. If this should work out the days of iPhones and Droids going the distance after a single juice up are not far off, nor are, for that matter, electric vehicles powered by powerful batteries.